Analysis of Vannamei shrimp DNA fragment resistant to White Spot Virus Syndrome
DOI:
https://doi.org/10.22219/ijota.v4i1.15839Keywords:
DNA fragment, DNA marker, PCR-RAPD, WSSVAbstract
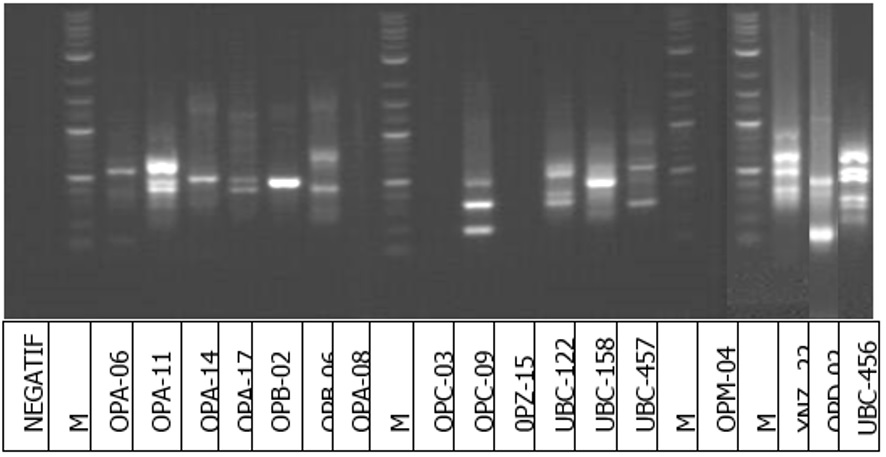
The attack of WSSV in Vannamei shrimp cultivation is still common. Shrimp quality improvement can be made through selection with the help of markers (marker-assisted choice). This study aimed to evaluate the DNA fragment profile of white shrimp that was resistant to WSSV disease. The analysis was performed using the PCR-RAPD method. WSSV challenged four groups of 100 Vannamei shrimp, then DNA was extracted from live and dead shrimp. The results showed that 2 of the 17 primers tested had high potential as markers, namely OP-09 and OPD-2. PCR products with OPC-09 primers had specific DNA bands measuring about 1.2 kb in all post-challenge WSSV resistant shrimp individuals. The amplification results using OPD-02 primers showed a particular band of DNA with a length of about 1.0 kb, with 60 % of the appearance in WSSV-resistant shrimp. In contrast, the WSSV-susceptible shrimp group did not have specific DNA fragments. Thus, the two RAPD primers had a high chance of being used in the selection with the help of markers to produce WSSV resistant shrimp.
Downloads
References
Browdy, C. L. (1998). Recent developments in penaeid broodstock and seed production technologies: improving the outlook for superior captive stocks. Aquaculture, 164(1-4): 3-21.
Chou, H., Huang, C., Wang, C., Chiang, H., & Lo, C. (1995). Pathogenicity of a baculovirus infection causing white spot syndrome in cultured penaeid shrimp in Taiwan. Dis Aquat Organ, 23(3): 165-173.
de Freitas, P. D., & Galetti, P. M. (2005). Assessment of the genetic diversity in five generations of a commercial broodstock line of Litopenaeus vannamei shrimp. African journal of biotechnology, 4(12): 1362-1367.
Hizer, S. E., Dhar, A. K., Klimpel, K. R., & Garcia, D. K. (2002). RAPD markers as predictors of infectious hypodermal and hematopoietic necrosis virus (IHHNV) resistance in shrimp (Litopenaeus stylirostris). Genome, 45(1): 1-7.
Jorde, L. B., Bamshad, M. J., Watkins, W. S., Zenger, R., Fraley, A. E., Krakowiak, P. A., Carpenter, K. D., Soodyall, H, Jenkins, T, & Rogers, A. R. (1995). Origins and affinities of modern humans: a comparison of mitochondrial and nuclear genetic data. American journal of human genetics 57(3): 523-538.
Khetpu, K. (2005). Genetic diversity of the blue swimming crab portunus pelagicus in Thailand analyzed by AFLP and RAPD. Chulalongkorn University.
Klinbunga, S., Amparyup, P., Tassanakajon, A., Jarayabhand P., & Yoosukh, W. (2000). Development of species-specific markers of the tropical oyster (Crassostrea belcheri) in Thailand. Marine Biotechnology, 2(5): 476-484.
Law, J. W. F., Ab Mutalib, N. S., Chan, K. G., & Lee, L. H. (2015). Rapid methods for the detection of foodborne bacterial pathogens: principles, applications, advantages and limitations. Front. Microbiol., 5: 770.
Mamiatis, T., Fritsch, E. F., Sambrook, J., & Engel, J. (1985). Molecular cloning–A laboratory manual. New York: Cold Spring Harbor Laboratory. 1982, 545 S., 42$. In: Wiley Online Library.
Matondang, I., Suharsono, Hartana, A. (2001). Genetic diversity analysis of tall coconut from Mollucas employing random amplified polymorphic DNA.
Migaud, H., Bell, G., Cabrita, E., McAndrew, B., Davie, A., Bobe, J., Harraez, M. P., & Carrillo, M. (2013). Gamete quality and broodstock management in temperate fish. Reviews in Aquaculture, 5: S194-S223.
Nakano, H., Koube, H., Umezawa, S., Momoyama, K., Hiraoka, M., Inouye, K., & Oseko, N. (1994). Mass mortalities of cultured kuruma shrimp, Penaeus japonicus, in Japan in 1993: epizootiological survey and infection trials. Fish Pathology, 29(2): 135-139.
Nguyen, N. H. (2016). Genetic improvement for important farmed aquaculture species with a reference to carp, tilapia and prawns in Asia: achievements, lessons and challenges. Fish Fisheries, 17(2): 483-506.
Nyonje, B., Opiyo, M., Orina, P., Abwao, J., Wainaina, M., & Charo-Karisa, H. (2018). Current status of freshwater fish hatcheries, broodstock management and fingerling production in the Kenya aquaculture sector. Livest. Res. Rural. Dev., 30: 1-8.
Rafalski, A., Tingey, S., & Willians, J. G. K. (1994). Random amplified polymorphic DNA (RAPD) markers. Plant Molecular Biology Manual, H4: 1-8.
Tingey, S., Rafalski, J., Williams, J. (1992). Genetic analysis with RAPD markers. ble. 3-8. Paper presented at the Proceedings of the Symposium, Applications of RAPD Technology to Plant Breeding. Crop Sci. Soc./Amer. Soc. Hort. Sci./Amer. Genet. Assoc.
Trang, T. T., Hung, N. H., Ninh, N. H., Knibb, W., & Nguyen, N. H. (2019). Genetic variation in disease resistance against White Spot Syndrome Virus (WSSV) in Liptopenaeus vannamei. Frontiers in genetics, 10: 264.
Triana, S. (2010). Analisis fragmen DNA ikan Kerapu Macan (Epinephelus fuscoguttatus) yang tahan dan rentan terhadap Bakteri Vibrio alginolyticus. Jurnal Ilmu Dasar, 11(1): 8-16.
Welsh J, McClelland M. 1990. Fingerprinting genomes using PCR with arbitrary primers. Nucleic acids research, 18(24): 7213-7218.
Williams, J. G., Kubelik, A. R., Livak, K. J., Rafalski, J. A., & Tingey, S. V. (1990). DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic acids research, 18(22): 6531-6535.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 IJOTA (Indonesian Journal of Tropical Aquatic)

This work is licensed under a Creative Commons Attribution-ShareAlike 4.0 International License.
IJOTA (Indonesian Journal of Tropical Aquatic) allows readers to read, download, copy, distribute, print, search, or link to its articles' full texts and allows readers to use them for any other lawful purpose. The journal allows the author(s) to hold the copyright without restrictions. Finally, the journal allows the author(s) to retain publishing rights without restrictions
- Authors are allowed to archive their submitted article in an open access repository
- Authors are allowed to archive the final published article in an open access repository with an acknowledgment of its initial publication in this journal

